which describes what will happen to the h+ when these reactants form products?
Overview of Acids and Bases
- Page ID
- 78247
At that place are three major classifications of substances known as acids or bases. The Arrhenius definition states that an acid produces H+ in solution and a base of operations produces OH-. This theory was adult by Svante Arrhenius in 1883. Afterwards, two more than sophisticated and general theories were proposed. These are the Brønsted-Lowry and the Lewis definitions of acids and bases. The Lewis theory is discussed elsewhere.
The Arrhenius Theory of Acids and Bases
In 1884, the Swedish chemist Svante Arrhenius proposed 2 specific classifications of compounds; acids and bases. When dissolved in an aqueous solution, certain ions were released into the solution. An Arrhenius acrid is a chemical compound that increases the concentration of H+ ions that are present when added to water. These H+ ions class the hydronium ion (HiiiO+) when they combine with water molecules. This process is represented in a chemical equation by adding H2O to the reactants side.
\[ HCl_{(aq)} \rightarrow H^+_{(aq)} + Cl^-_{(aq)} \]
In this reaction, hydrochloric acid (\(HCl\)) dissociates completely into hydrogen (H+) and chlorine (Cl-) ions when dissolved in h2o, thereby releasing H+ ions into solution. Formation of the hydronium ion equation:
\[ HCl_{(aq)} + H_2O_{(l)} \rightarrow H_3O^+_{(aq)} + Cl^-_{(aq)} \]
The Arrhenius theory, which is the simplest and least general description of acids and bases, includes acids such equally HClO4 and HBr and bases such as \(NaOH\) or \(Mg(OH)_2\). For example the complete dissociation of \(HBr\) gas into h2o results generates costless \(H_3O^+\) ions.
\[HBr_{(g)} + H_2O_{(l)} \rightarrow H_3O^+_{(aq)} + Br^-_{(aq)}\]
This theory successfully describes how acids and bases react with each other to make water and salts. However, it does non explain why some substances that do not comprise hydroxide ions, such as \(F^-\) and \(NO_2^-\), can make basic solutions in water. The Brønsted-Lowry definition of acids and bases addresses this problem.
An Arrhenius base is a compound that increases the concentration of OH- ions that are present when added to h2o. The dissociation is represented by the following equation:
\[ NaOH \; (aq) \rightarrow Na^+ \; (aq) + OH^- \; (aq) \]
In this reaction, sodium hydroxide (NaOH) disassociates into sodium (Na+) and hydroxide (OH-) ions when dissolved in water, thereby releasing OH- ions into solution.
Annotation
- Arrhenius acids are substances which produce hydrogen ions in solution.
- Arrhenius bases are substances which produce hydroxide ions in solution.
Free Hydrogen Ions do not Exist in H2o
Owing to the overwhelming excess of \(H_2O\) molecules in aqueous solutions, a bare hydrogen ion has no chance of surviving in h2o. The hydrogen ion in aqueous solution is no more than a proton, a bare nucleus. Although it carries only a single unit of positive accuse, this charge is full-bodied into a volume of infinite that is only about a hundred-millionth every bit large as the volume occupied by the smallest atom. (Call up of a pebble sitting in the middle of a sports stadium!) The resulting extraordinarily high charge density of the proton strongly attracts it to any part of a nearby cantlet or molecule in which there is an excess of negative charge. In the case of water, this volition be the solitary pair (unshared) electrons of the oxygen atom; the tiny proton will be buried inside the lone pair and will course a shared-electron (coordinate) bond with it, creating a hydronium ion, \(H_3O^+\). In a sense, \(H_2O\) is acting every bit a base here, and the product \(H_3O^+\) is the conjugate acid of water:
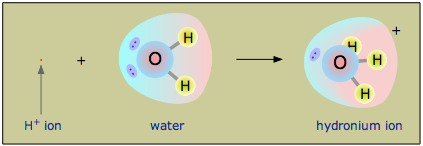
Although other kinds of dissolved ions accept water molecules jump to them more or less tightly, the interaction between H+ and \(H_2O\) is then potent that writing "H+ (aq) " inappreciably does information technology justice, although it is formally correct. The formula \(H_3O^+\) more than adequately conveys the sense that information technology is both a molecule in its own correct, and is also the conjugate acid of water.
The equation "HA → H+ + A–" is so much easier to write that chemists withal use it to represent acid-base reactions in contexts in which the proton donor-acceptor machinery does not demand to be emphasized. Thus, it is permissible to talk about "hydrogen ions" and utilise the formula H+ in writing chemical equations as long as y'all remember that they are not to be taken literally in the context of aqueous solutions.
Limitations to the Arrhenius Theory
The Arrhenius theory has many more limitations than the other ii theories. The theory suggests that in society for a substance to release either H+ or OH- ions, it must incorporate that particular ion. Notwithstanding, this does non explicate the weak base ammonia (NH3) which, in the presence of water, releases hydroxide ions into solution, simply does not incorporate OH- itself.
Hydrochloric acid is neutralized by both sodium hydroxide solution and ammonia solution. In both cases, you go a colourless solution which you lot tin crystallize to get a white salt - either sodium chloride or ammonium chloride. These are clearly very similar reactions. The full equations are:
\[ NaOH \; (aq) + HCl \; (aq) \rightarrow NaCl \; (aq) + H_2O \; (l) \]
\[ NH_3 \; (aq) + HCl \; (aq) \rightarrow NH_4Cl \; (aq) \]
In the sodium hydroxide case, hydrogen ions from the acid are reacting with hydroxide ions from the sodium hydroxide - in line with the Arrhenius theory. Yet, in the ammonia example, there are no hydroxide ions!
You lot can get around this by saying that, when the ammonia reacts with the h2o, it is dissolved in to produce ammonium ions and hydroxide ions:
\[ NH_3 \; (aq) + H_2O \; (l) \rightleftharpoons NH_4^+ \; (aq) + OH^- \;(aq) \]
This is a reversible reaction, and in a typical dilute ammonia solution, about 99% of the ammonia remains as ammonia molecules. Nevertheless, there are hydroxide ions in that location, and we can squeeze this into the Arrhenius theory. However, this same reaction also happens between ammonia gas and hydrogen chloride gas.
\[ NH_3 \; (g) + HCl \; (chiliad) \rightarrow NH_4Cl \;(south) \]
In this case, there are non whatever hydrogen ions or hydroxide ions in solution - considering there isn't whatsoever solution. The Arrhenius theory wouldn't count this as an acid-base reaction, despite the fact that information technology is producing the same product as when the ii substances were in solution. Because of this shortcoming, later theories sought to improve explicate the behavior of acids and bases in a new manner.
The Brønsted-Lowry Definition
In 1923, chemists Johannes Nicolaus Brønsted and Thomas Martin Lowry independently adult definitions of acids and bases based on the compounds' abilties to either donate or accept protons (H+ ions). In this theory, acids are defined as proton donors; whereas bases are divers as proton acceptors. A compound that acts as both a Brønsted-Lowry acid and base of operations together is chosen amphoteric.This took the Arrhenius definition i stride further, every bit a substance no longer needed to be composed of hydrogen (H+) or hydroxide (OH-) ions in gild to be classified as an acid or base. Consider the following chemical equation:
\[ HCl \; (aq) + NH_3 \; (aq) \rightarrow NH_4^+ \; (aq) + Cl^- \; (aq) \]
Here, hydrochloric acid (HCl) "donates" a proton (H+) to ammonia (NHiii) which "accepts" information technology , forming a positively charged ammonium ion (NH4 +) and a negatively charged chloride ion (Cl-). Therefore, HCl is a Brønsted-Lowry acrid (donates a proton) while the ammonia is a Brønsted-Lowry base of operations (accepts a proton). Besides, Cl- is chosen the conjugate base of operations of the acrid HCl and NH4 + is called the conjugate acid of the base NH3.
The Brønsted-Lowry Theory of Acids and Bases
- A Brønsted-Lowry acrid is a proton (hydrogen ion) donor.
- A Brønsted-Lowry base is a proton (hydrogen ion) acceptor.
In this theory, an acid is a substance that can release a proton (like in the Arrhenius theory) and a base of operations is a substance that tin can accept a proton. A basic salt, such as Na+F-, generates OH- ions in water past taking protons from water itself (to make HF):
\[F^-_{(aq)} + H_2O_{(fifty)} \rightleftharpoons HF_{(aq)} + OH^-\]
When a Brønsted acid dissociates, information technology increases the concentration of hydrogen ions in the solution, \([H^+]\); conversely, Brønsted bases dissociate by taking a proton from the solvent (water) to generate \([OH^-]\).
- Acid dissociation
\[HA_{(aq)} \rightleftharpoons A^-_{(aq)} + H^+_{(aq)}\]
- Acid Ionization Abiding:
\[K_a=\dfrac{[A^-][H^+]}{[HA]}\]
- Base dissociation:
\[B_{(aq)} + H_2O_{(fifty)} \rightleftharpoons HB^+_{(aq)} + OH^-_{(aq)}\]
- Base Ionization Abiding
\[K_b = \dfrac{[HB^+][OH^-]}{[B]}\]
Conjugate Acids and Bases
One important result of these equilibria is that every acid (\(HA\)) has a conjugate base (\(A^-\)), and vice-versa. In the base of operations, dissociation equilibrium in a higher place the cohabit acrid of base \(B\) is \(HB^+\). For a given acrid or base, these equilibria are linked by the water dissociation equilibrium:
\[H_2O_{(l)} \rightleftharpoons H^+_{(aq)} + OH^-_{(aq)}\]
with
\[K_w = [H^+][OH^-]\]
for which the equilibrium constant Kw is i.00 ten 10-xiv at 25°C. It can be easily shown that the product of the acrid and base dissociation constants Ka and Kb is Gw.
Potent and Weak Acids and Bases
Strong acids are molecular compounds that essentially ionize to completion in aqueous solution, disassociating into H+ ions and the additional anion; there are very few mutual strong acids. All other acids are "weak acids" that incompletely ionized in aqueous solution. Acids and bases that dissociate completely are said to be strong acids, e.g.:
- \(HClO_{four(aq)} \rightarrow H^+_{(aq)} + ClO^-_{four(aq)}\)
- \(HBr_{(aq)} \rightarrow H^+_{(aq)} + Br^-_{(aq)}\)
- \(CH_3O^-_{(aq)} + H_2O_{(l)} \rightarrow CH_3OH_{(aq)} + OH^-_{(aq)}\)
- \(NH^-_{2(aq)} + H_2O_{(l)} \rightarrow NH_{3(aq)} + OH^-_{(aq)}\)
Here the right-handed arrow (\(\rightarrow\)) implies that the reaction goes to completion. That is, a 1.0 Chiliad solution of HClO4 in water actually contains 1.0 M H+(aq) and 1.0 M ClO4 -(aq), and no undissociated HClO4.
Conversely, weak acids such as acetic acid (CH3COOH) and weak bases such as ammonia (NH3) dissociate simply slightly in h2o - typically a few percent, depending on their concentration and be by and large as the undissociated molecules.
- Strong ACIDS: HCl, HNO3, H2SO4, HBr, HI, HClOfour
- WEAK ACIDS: All other acids, such equally HCN, HF, H2S, HCOOH
Strong acids such every bit \(HCl\) dissociate to produce spectator ions such as \(Cl^-\) every bit cohabit bases, whereas weak acids produce weak cohabit bases. This is illustrated below for acerb acrid and its conjugate base, the acetate anion. Acetic acid is a weak acid (Ka = 1.8 x 10-five) and acetate is a weak base (Kb = Kw/Yarda = 5.half dozen x ten-10)

Similar acids, potent and weak bases are classified by the extent of their ionization. Potent bases disassociate almost or entirely to completion in aqueous solution. Similar to strong acids, there are very few common stiff bases. Weak bases are molecular compounds where the ionization is not consummate.
- STRONG BASES: The hydroxides of the Grouping I and Grouping Two metals such every bit LiOH, NaOH, KOH, RbOH, CsOH
- WEAK BASES: All other bases, such as NH3, CHthreeNH2, C5H5N
Note
The forcefulness of a conjugate acrid/base of operations varies inversely with the strength or weakness of its parent acid or base. Any acrid or base is technically a conjugate acrid or conjugate base too; these terms are simply used to identify species in solution (i.due east acetic acid is the conjugate acid of the acetate anion, a base, while acetate is the cohabit base of acerb acid, an acid).
How does one ascertain acids and bases? In chemistry, acids and bases have been defined differently past 3 sets of theories. One is the Arrhenius definition, which revolves around the idea that acids are substances that ionize (break off) in an aqueous solution to produce hydrogen (H+) ions while bases produce hydroxide (OH-) ions in solution. On the other hand, the Brønsted-Lowry definition defines acids equally substances that donate protons (H+) whereas bases are substances that have protons. Also, the Lewis theory of acids and bases states that acids are electron pair acceptors while bases are electron pair donors. Acids and bases tin exist defined by their physical and chemic observations.
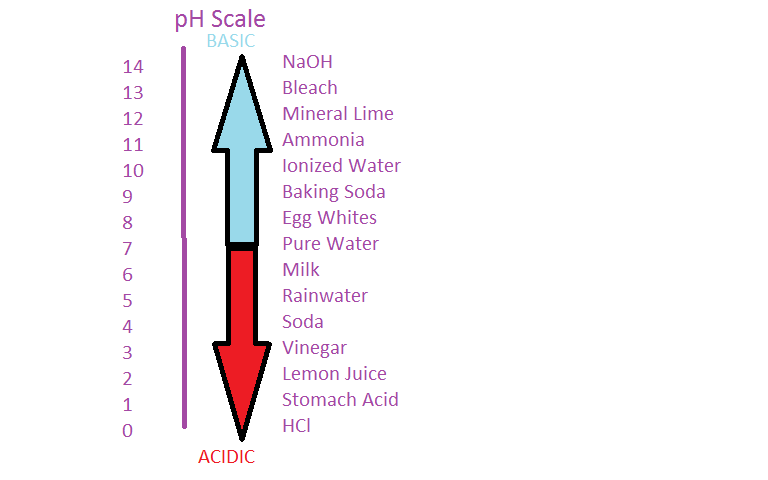
pH Calibration
Since acids increment the amount of H+ ions nowadays and bases increase the corporeality of OH- ions, under the pH scale, the force of acerbity and basicity can be measured by its concentration of H+ ions. This scale is shown past the following formula:
pH = -log[H+]
with [H+] existence the concentration of H+ ions.
To see how these calculations are done, refer to Calculating the pH of the solution of a Polyprotic Base/Acid
The pH scale is often measured on a ane to xiv range, but this is wrong (see pH for more details). Something with a pH less than seven indicates acidic properties and greater than vii indicates bones properties. A pH at exactly 7 is neutral. The higher the [H+], the lower the pH.
Lewis Theory
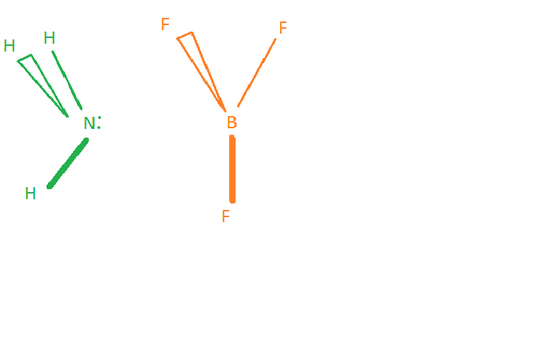
The Lewis theory of acids and bases states that acids human activity as electron pair acceptors and bases human action every bit electron pair doners. This definition doesn't mention annihilation near the hydrogen cantlet at all, dissimilar the other definitions. It only talks almost the transfer of electron pairs. To demonstrate this theory, consider the following example.
This is a reaction between ammonia (NH3) and boron trifluoride (BFthree). Since at that place is no transfer of hydrogen atoms hither, information technology is clear that this is a Lewis acrid-base reaction. In this reaction, NH3 has a alone pair of electrons and BFthree has an incomplete octet, since boron doesn't have enough electrons around it to form an octet.
Because boron just has 6 electrons around it, it tin can hold 2 more than. BF3 tin can human action as a Lewis acid and accept the pair of electrons from the nitrogen in NH3 , which will and then form a bail between the nitrogen and the boron.
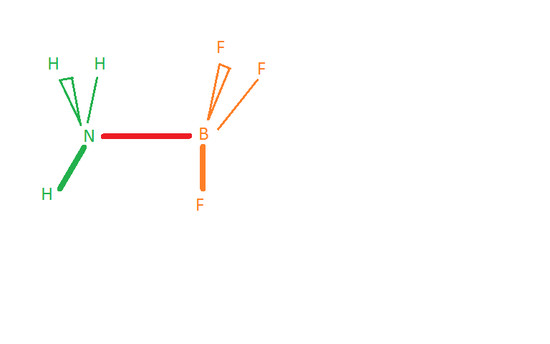
This is considered an acid-base reaction where NH3 (base) is donating the pair of electrons to BFiii. BFthree (acid) is accepting those electrons to form a new chemical compound, HiiiNBFthree.
Neutralization
A special property of acids and bases is their ability to neutralize the other'due south backdrop. In an acid-base of operations (or neutralization) reaction, the H+ ions from the acrid and the OH- ions from the base of operations react to create h2o (HtwoO). Another product of a neutralization reaction is an ionic compound chosen a salt. Therefore, the full general form of an acid-base of operations reaction is:
The post-obit are examples of neutralization reactions:
ane.
(Annotation: To see this reaction washed experimentally, refer to the YouTube video link under the section "References".)
2.
Titrations
Titrations are performed with acids and bases to determine their concentrations. At the equivalence point, the number of moles of the acid will equal the number of moles of the base of operations. This indicates that the reaction has been neutralized.
Neutralization: moles of acrid = moles of base of operations
Here's how the calculations are done:
For instance, muriatic acid is titrated with sodium hydroxide:
For instance, 30 mL of one.00 Thousand NaOH is needed to titrate 60 mL of an HCl solution. The concentration of HCl needs to exist determined. At the eqivalence point:
moles of HCl = moles of NaOH
To solve for the molarity of HCl, plug in the given data into the equation above.
ChiliadHCl(60 mL HCl) = (1.00 M NaOH)(thirty mL NaOH)
MHCl=0.5 1000
The concentration of HCl is 0.5 K.
Sample Problems
one. Which of the following compounds is a strong acid?
- CaSO4
- NaCl
- HNO3
- NHiii
Solution: There are 6 potent acids and all other acids are considered weak. HNO3 is ane of those six strong acids, while NH3 is actuallly a weak base.
The respond is (iii) HNOiii .
2. Which of the post-obit compounds is a Brønsted-Lowry base?
- HCl
- HPO4 2 -
- H3POfour
- NH4 +
- CH3NH3 +
Solution: A Brønsted-Lowry Base of operations is a proton acceptor, which ways information technology will accept in an H+. This eliminates HCl, HiiiPO4 ,NH4 + and CH3NH3 + because they are Brønsted-Lowry acids. They all give away protons. In the example of HPO4 2-, consider the following equation:
Here, it is articulate that HPO4 2 - is the acrid since it donates a proton to water to brand H3O+ and PO4 3 -. Now consider the following equation:
In this case, HPO4 ii - is the base since information technology accepts a proton from water to form H2PO4 - and OH-. Thus, HPO4 2 - is an acrid and base of operations together, making it amphoteric.
Since HPO4 2 - is the simply compound from the options that can deed as a base, the answer is (ii) HPO4 2-.
3. A l ml solution of 0.five 1000 NaOH is titrated until neutralized into a 25 ml sample of HCl. What was the concentration of the HCl?
Solution: Since the number of moles of acrid equals the number of moles of base of operations at neutralization, the following equation is used to solve for the molarity of HCl:
Now, plug into the equation all the information that is given:
MHCl(25 mL HCl) = (0.5 MNaOH)(50 mL NaOH)
ThouHCl = 1
The correct answer is i MHCl .
four. In the following acrid-base neutralization, 2.79 g of the acrid HBr (80.91g/mol) neutralized 22.72 mL of a bones aqueous solution by the reaction:
Calculate the molarity of the bones solution.
Solution:
First, the number of moles of the acrid needs to exist calculated. This is done by using the molar mass of HBr to convert 2.79 1000 of HBr to moles.
(2.79 g HBr)/(80.91 m/mol HBr) = 0.0345 moles HBr
Since this is a neutralization reaction, the number of moles of the acid (HBr) equals the number of moles of the base (NaOH) at neutralization:
moles of acid = moles of base
0.0345 moles HBr = 0.0345 moles NaOH
The molarity of NaOH tin now be determined since the amount of moles are plant and the book is given. Convert 22.72 mL to Liters first since molarity is in units of moles/Fifty.
Molarity = (0.0345 moles NaOH)/(0.02272 L NaOH) = 1.52 1000NaOH
The right answer is 1.52 YardNaOH .
5. Which of the following is a Brønsted-Lowry base but not an Arrhenius base of operations?
- NH3
- NaOH
- Ca(OH)2
- KOH
Solution: The Brønsted-Lowry definition says that a base accepts protons (H+ ions). NaOH, Ca(OH)2, and KOH are all Arrhenius bases because they yield the hydroxide ion (OH-) when they ionize. Yet, NH3 does not dissociate in water like the others. Instead, it takes a proton from h2o and becomes NH4 while water becomes a hydroxide.
Therefore, the correct answer is (i) NH3 .
References
-
Brent, Lynnette. Acids and Bases. New York, NY: Crabtree Pub., 2009. Print.
-
Hulanicki, Adam. Reactions of Acids and Bases in Analytical Chemistry. Ellis Horwood Express: 1987.
-
Oxlade, Chris. Acids & Bases. Chicago, IL: Heinemann Library, 2002. Impress.
- Petrucci, Ralph H. General Chemistry: Principles and Mod Applications. Macmillian: 2007.
- Vanderwerd, Calvin A. Acids, Bases, and the Chemistry of the Covalent Bond. Reinhold: 1961.
Contributors and Attributions
- Catherine Broderick (UCD), Marianne Moussa (UCD)
-
Jim Clark (Chemguide.co.uk)
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acid/Overview_of_Acids_and_Bases
Post a Comment for "which describes what will happen to the h+ when these reactants form products?"